
At first glance, Electromigration (EM) and Electrochemical Migration (ECM) might seem alike. In both cases, metal atoms or ions move from a high-voltage area to a low-voltage area, and both can lead to dendrite growth. But they’re actually very different at their core.
Electromigration is a physical process where flowing electrons push metal atoms along a path. Over time, this can thin out parts of a wire and cause open circuits or shorts.
Electrochemical migration, on the other hand, is a chemical process. It happens when metal ions move and build up into conductive bridges under the influence of moisture and electric fields. This usually results in short circuits.
Electromigration (EM):
Electromigration happens when metal atoms inside a wire get pushed along by the flow of electrons. This usually occurs in very thin wires, like the ones found in high-density electronic circuits such as transistors. As current flows, the moving electrons bump into the metal atoms and slowly push them in the direction of the current. Over time, this can cause parts of the wire to thin out or even break completely, which can lead to circuit failure.
Think of a tiny metal wire inside a microchip. As current flows through it, electrons keep colliding with the metal atoms, nudging them from one end of the wire to the other. In spots where the wire is narrower and the current is stronger, the effect is even more intense. Eventually, the wire might wear down in one area and crack, causing an open circuit—or material might build up in another area and cause a short.
It’s kind of like how a river gradually erodes soil from one bank and carries it downstream. Even though electromigration is driven by electron collisions instead of water flow, the idea of material being slowly moved over time is pretty similar.
Electromigration doesn’t happen overnight, but it can seriously affect the reliability of small electronic parts. That’s why engineers take it into account when designing devices—choosing the right materials and wire sizes to make sure things stay stable over time.
Key Factors that Affect Electromigration:
-
Presence of mobile metal atoms
-
High current density
-
(Optional) High temperatures – Elevated temperatures increase atom movement and speed up EM. That’s why thermal control is critical in electronics design. Using low-temperature materials and thermal management techniques can help mitigate EM.
It’s worth noting that humidity and environmental moisture do not affect electromigration, because EM happens inside the metal junctions, not on the surface.
Electrochemical Migration (ECM):
Electrochemical Migration (ECM) happens when metal ions from a conductor or plating layer move across the surface of a conductor or an electrolyte under the influence of a voltage bias and moisture. This creates a chemical environment where electrolytes form, allowing metal ions to migrate. As these ions move, they often create unintended conductive paths that most commonly result in short circuits. In some cases, they may also damage insulation layers, but the primary concern with ECM is its tendency to cause electrical shorts that can lead to component failure.
ECM typically shows up in humid environments, like areas with high moisture levels or damp industrial settings. When moisture combines with electrical bias, it can trigger chemical reactions that cause metal ions to move from one area to another—often between conductors or between a conductor and an insulator. These migrating ions can eventually form conductive bridges, resulting in electrical shorts or system failures.
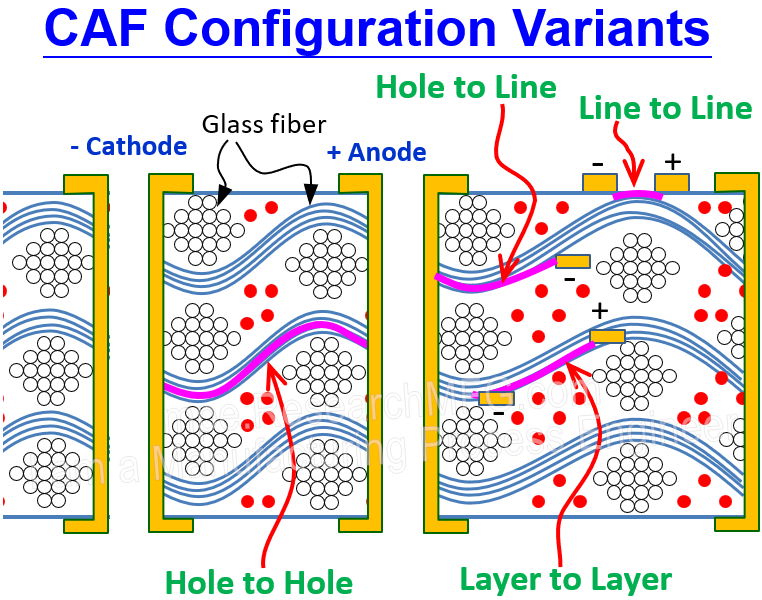
One of the most common examples of ECM is Conductive Anodic Filament (CAF) failure in printed circuit boards (PCBs). If a PCB is exposed to high humidity and a DC voltage is applied, moisture can mix with impurities and create electrolytes. These electrolytes may enter tiny gaps between PCB layers (layer-to-layer), traces (line-to-line), plated holes (hole-to-hole), or from holes to traces (hole-to-line). At the anode—the positive side—copper can oxidize into Cu+ or Cu++ ions. These ions then migrate along the fiberglass bundles inside the PCB toward the cathode. At the same time, free electrons travel from the cathode to the anode. When the copper ions meet these electrons, they’re reduced back into copper metal. This oxidation and reduction cycle repeats over and over, gradually forming a copper bridge—or dendrite—stretching from the anode to the cathode. Eventually, this leads to a short circuit.
Several factors can influence the ECM process, including humidity, the presence of materials that can generate ions, and the strength of the voltage bias. That’s why engineers need to plan for ECM prevention when designing and manufacturing electronic products.
Key Factors That Affect Electrochemical Migration:
-
Moisture or high humidity – Required to form electrolytes.
-
Presence of mobile metal ions – Common ions involved in ECM include silver (Ag), copper (Cu), palladium (Pd), and tin (Sn), listed in order of how fast they form dendrites.
-
High bias voltage – Drives ion migration.
-
(Optional) High temperature – Can also accelerate the ECM process.
When a positive voltage is applied, metal cations (Mn⁺) in a contaminated moisture environment move toward the cathode (negative side). Once there, they undergo a reduction reaction (Mn⁺ + ne⁻ → M) and begin to deposit. Over time, these metal deposits form needle-like or tree-shaped structures called dendrites, which grow from the cathode back toward the anode. These dendrites are the most visible sign of ECM and a major cause of electrical shorts.
YouTube: Electrochemical Migration (ECM) – Dendrite formation / Dendritic growth
Related Posts:








Leave a Reply